Ask Question Asked 6 years 5 months ago. Incompatible Materials Strong oxidizing agents Strong bases Hazardous Decomposition ProductsCarbon monoxide CO Carbon dioxide CO2 Hydrogen halides.
1 Bromo 2 Methyl 2 Propanamine C4h10brn Chemspider
Articles of 2-methylpropan-2-ol are included as well.

. Dayang Chem Hangzhou Co Ltd. More than 16kg with plastic bag insidefiber drum outside according to the extract quantity. 1-bromo-2-methylpropane - cas 78-77-3 synthesis structure density melting point boiling point.
It is not necessary to draw lone pair electrons. 1 b Name and outline the mechanism for the formation of 3-bromo-3-methylpentane from this reaction of 3-methylpent-2-ene with hydrogen bromide. Exposure controlspersonal protection Control parameters.
Choose all the intermolecular interactions that could occur in a container of only methyl benzoate. 2-methylpropan-2-amine hydrochloride 2-Amino-2-methylpropane hydrochloride 11-dimethylethylammonium chloride Top Suppliers. The electrophile is a tertiary carbocation so that rules out sn2.
CH 3 2 CHCH 2 Br. IName and outline a mechanism for the conversion of 2-bromo-3-methylbutane into 2-methylbut-2-ene according to the equation. Major products of the reaction of 2-bromo-2-methylpropane and propanoic acid.
It is not necessary to draw lone pair electrons. Modified 6 years 5 months ago. WEB SEARCH MSDS RESOURCES SUPPLIERS.
Draw the structure for 3-ethoxypentane. 4 Related Records Expand this section. 1 Reaction Mechanism for Tertiary Halogenoalkanes An example of a tertiary halogenoalkane is 2-bromo-2-methylpropane.
Find 2-methyl-2-propanamine and related products for scientific research at MilliporeSigma. I believe the nucleophile is a rather weak base since carboxylic acids are. I want be here.
Keep away from heat hot surfaces sparks open flames and other ignition sources. 2-Bromo-2-methylpropane Revision Date 24-Dec-2021 Conditions to Avoid Keep away from open flames hot surfaces and sources of ignition. Viewed 2k times 1 begingroup Heres my attempt.
1-5kg with ziplock plastic bag insideAluminum foil bag outsidefinally with waterproof bag. 2-Methylpropan-1-ol can be prepared by reacting 1-bromo-2-methylpropane with dilute aqueous sodium hydroxide. C 4 H 11 N.
CID 426399 1-Bromo-2-methylpropan-2-amine Dates. Binduced dipoleinduced dipole interactions ie London dispersion forces Cdipoledipole interactions. Highly flammable liquid and vapour.
Empirical Formula Hill Notation. Name of mechanism Nucleophilic Substitution Mechanism 3 b When 20 cm 3 of 1-bromo-2-methylpropane M r 1369 were reacted with an excess of sodium hydroxide 895 mg of 2. With 4-bromo-N-phenyl-piperidine-1-carboxamideN-methylpropan-2-a to form 4-isopropyl-methyl-amino-N-phenyl-piperidine-1-carboxamide.
A Name and outline the mechanism for this reaction. The density of 1-bromo-2-methylpropane is 126 g cm3 Calculate the percentage yield for this reaction. Chemsrc provides 2-methylpropan-2-olCAS1093099-09-2 MSDS density melting point boiling point structure formula molecular weight etc.
C 10 H 14 ClN. 2 Names and Identifiers Expand this section. Draw the structure of Z-3-methylpent-2-ene.
R 1369 were reacted with an excess of sodium hydroxide 895 mg of 2-methylpropan-1-ol Mr 740 were obtained. 3 Chemical and Physical Properties Expand this section. 3DPX-001556 2-bromobutane James Tyrwhitt Drake 2-Bromobutane SEC-BUTYL BROMIDE Butane 2-bromo- 78-76-2 2-Butyl bromide Methylethylbromomethane 2-Bromo-butane sec-Butylbromide 1-Bromo-1-methylpropane 2-Bromo butane.
The major product is 2-methylbut-2-ene. When 2-bromo-2-methylpropane is refluxed with an aqueous solution of sodium hydroxide the nucleophilic hydroxide ion substitutes the bromine of the tertiary halogenoalkane to form the tertiary alcohol 2-methylpropan-2-ol. A part from the uses mentioned in section 12 no other specific uses are stipulated SECTION 8.
6-15kg with plastic bag insideAluminum foil bag outsidefinally with carton box. Induced dipoleinduced dipole interactions ie London dispersion forces 4. Up to 24 cash back a Hot concentrated ethanolic potassium hydroxide reacts with 2-bromo-3-methylbutane to form two alkenes that are structural isomers of each other.
CAS 78-77-3 chemical formula CH₃₂CHCH₂Br. Explain why more 3-bromo-3-methylpentane. Draw the structure for 3-ethoxypentane.
Click here for details. 1 Structures Expand this section. 12-Dibromoethane BrCH22Br or C2H4Br2 CID 7839 - structure chemical names physical and chemical properties classification patents literature biological.
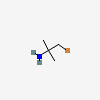
1 Bromo 2 Methylpropan 2 Amine C4h10brn Pubchem
1 Bromo 2 Methylpropan 2 Amine Hydrobromide In Stock

1 Bromo 2 Methylpropane 78 77 3 C4h9br Density Melting Point Boiling Point Structural Formula Synthesis
13892 97 2 1 Bromo 2 Methylpropan 2 Amine Cas No 13892 97 2 1 Bromo 2 Methylpropan 2 Amine
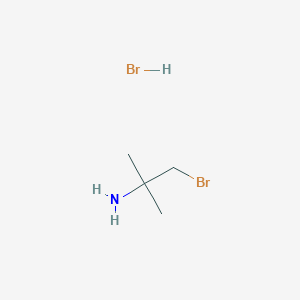
1 Bromo 2 Methylpropan 2 Amine Hydrobromide C4h11br2n Pubchem

1 Bromo 2 Methylpropane Structure C4h9br Over 100 Million Chemical Compounds Mol Instincts

Solved 1 Draw 1 Bromo 2 Methylpropan 2 Amine 2 Draw Chegg Com

0 comments
Post a Comment